Answer:

Step-by-step explanation:
First, we need to find the molecular mass of water (H₂O).
H₂O has:
- 2 Hydrogen atoms (subscript of 2)
- 1 Oxygen atom (implied subscript of 1)
Use the Periodic Table to find the mass of hydrogen and oxygen. Then, multiply by the number of atoms of the element.
- Hydrogen: 1.0079 g/mol
- Oxygen: 15.9994 g/mol
There are 2 hydrogen atoms, so multiply the mass by 2.
- 2 Hydrogen: (1.0079 g/mol)(2)= 2.0158 g/mol
Now, find the mass of H₂O. Add the mass of 2 hydrogen atoms and 1 oxygen atom.
- 2.0158 g/mol + 15.9994 g/mol = 18.0152 g/mol
Next, find the amount of moles using the molecular mass we just calculated. Set up a ratio.
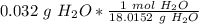
Multiply. The grams of H₂O will cancel out.
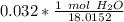
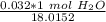
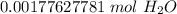
The original measurement given had two significant figures (3,2). We must round to have 2 significant figures. All the zeroes before the 1 are not significant. So, round to the ten thousandth.
The 7 in the hundred thousandth place tells us to round up.
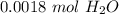
There are about 0.0018 moles in 0.032 grams.