Answer:
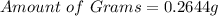
Step-by-step explanation:
Given
Element: Sodium
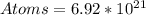
Required
Determine the amount of grams
We start by solving for the number of moles
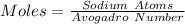
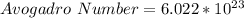
So, we have:
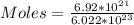
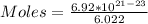
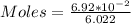
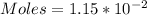 moles
moles
Next, we calculate the amount of grams.
Mass per mol of Sodium = 22.98977g/mol
For
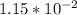 moles of sodium
moles of sodium

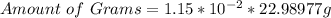
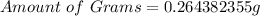
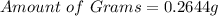 (approximated)
(approximated)
Hence, there are 0.2644g of sodium in
 atoms
atoms