Answer:
0.812 M
Step-by-step explanation:
The reaction that takes place is:
First we convert grams of Ca(NO₃)₂ to moles, using its molecular weight:
- 20.0 g Ca(NO₃)₂ ÷ 164.088 g/mol = 0.122 mol Ca(NO₃)₂
Then we convert Ca(NO₃)₂ moles to NO₃⁻, keeping in mind the stoichiometry of the reaction:
- 0.122 moles Ca(NO₃)₂ *
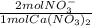 = 0.244 mol NO₃⁻
= 0.244 mol NO₃⁻
Finally we divide the number of moles by the volume (in L), to calculate the concentration:
(300.0 mL ⇒ 300.0 / 1000 = 0.300 L)
- Molarity = 0.244 mol NO₃⁻ / 0.300 L = 0.812 M